Deep Learning for Microscopy Image Analysis#
Lab authors: Damian Dalle Nogare, Florian Jug, and Beth Cimini .
This file last updated 2024-04-08.
Learning Objectives#
Appreciate how Neural Networks are trained
Segmentation with Cellpose9
Learn how to get to and use Google Colab
Denoising with Noise2Void10 in “Zero”
Bonus: Try in-browser classification with Piximi
Bonus: Use Noise2Void in Fiji
Bonus: Segmentation with StarDist in “Zero”
Lab Data: https://tinyurl.com/qi2024labs
Overview#
Neural networks can do useful things. Their deployment within user-friendly tools is, unfortunately, lagging behind. Hence, methods we would like to apply to our data are not always available in Fiji or ilastik8 quite yet (or only with a fair amount of command line work and difficulty). The latest methods can only be used by the ones “brave” enough to expose themselves to some amount of computer source code…
Today we will all be brave! 😊
I’m very much looking forward to hearing about your successes and struggles tomorrow during the Q&A session. Now, please take a seat, open a browser, and buckle up.
Exercise 1: Remind yourself about what we’ve heard in the lecture#
Visit https://playground.tensorflow.org and look around. What terms did you hear before, what is new, and what is confusing?
Please try to:
a. On the classification example that looks like a tiny checkerboard, try to get a test loss of 0.001 or less.

b. For the spiral-shaped classification example, try to find a network architecture with the smallest amount of nodes (neurons) that will drop below 0.01 (1%) test error.
c. Now switch from ‘Classification’ to ‘Regression’. What is going on here? Can you figure out how regression is different from classification?

d. Some other things to try if you feel it…
Add some noise to your data. What changes? Why?
Try to find a setup that overfits. How do you identify overfitting?

e. All the important terms and concepts wrt. to training and validation are somewhere on this one page. Check if there is anything that makes no sense to you and ask us
Exercise 2: Play with CellPose#
Note
Once back home, you will need this link to get started: https://cellpose.readthedocs.io/en/latest/installation.html
Here, at QI, we have taken this annoying step for you already. Hence, you will find Cellpose pre-installed on the lab computers. To start it, go to the Windows search next to the start menu, and type “anaconda”. Pick and start the option “anaconda prompt”.
Once this is opened, type in those two comments (the stuff after “>”):
> conda activate cellpose
(cellpose) > cellpose
You should now see something like
this: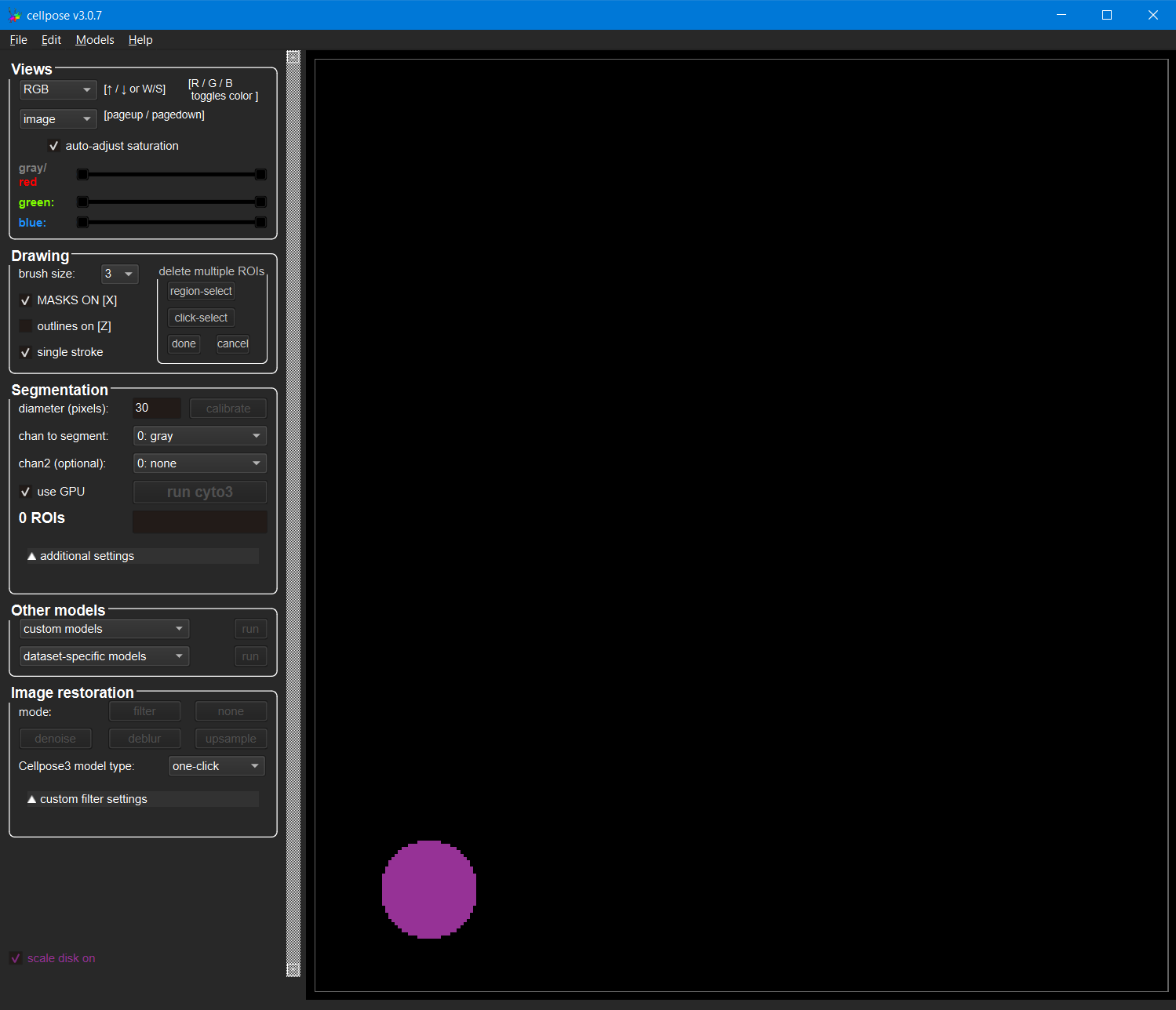
Open the file easy\001_img.tif by dragging it onto the open window.
You can find this file in the folder ‘DL4MIA’ in the Lab Data share, or
download it directly via
https://drive.google.com/drive/folders/1BgoUf1f-QfcFNIsppdzTCjehkCAntbyc?usp=share_link
We need to tell cellpose (roughly) how large our objects are (you can do
so via the cell diameter) field. How might we estimate this? Keep in
mind that this diameter must be reported in pixels.
You can now segment this image by selecting one of the pre-trained
models from within the model zoo box. Try segmenting this image using
the cyto model. How good are the results?
Tip
You can toggle the visibility of segmentation masks on and off
by hitting ‘x’ on your keyboard. Similarly, you can toggle cell
outlines with the keyboard shortcut ‘z’. Alternatively you can do so
in the Drawing tab.
How well did cellpose segment your image? Where (if anywhere) did it fail? Try some different models from the model zoo box. Do any of these work better? Worse? Why might that be?
Let’s now try some more challenging data. From the link above, or from the DL4MIA folder in the lab data share, download the entire folder named ‘hard’, and place it somewhere convenient (like the desktop).
From within this folder, open the ‘test’ folder and drag the file test_img.tif to cellpose to open it. Try segmenting it as well as possible. Can you find setting and a model that work perfectly?
Spoiler, none of the models are perfectly suited to this data, but we can iteratively retrain a model from within the Cellpose GUI… interested? Ok, let’s do it! 🙂
Human-in-the-loop retraining#
In the ‘hard’ folder you download earlier you will find a folder called ‘train’. In this folder you will find a number of images. Open the image 104_img.tif’ in cellpose. Note that we are not going to train a model from scratch, instead we are going to finetune one of the existing models (ideally starting from one that does a pretty good job already). Choose the model that you think gave you the best segmentations in the previous part of this exercise and apply it to this image.
We are going to iteratively finetune this model, one image at a time. Once the current image is segmented well, we will open another one and repeat until results are (hopefully) making us happy! Here a little sketch:
Everytime we see a result, you can correct the segmentation errors by redrawing some of the segmentation masks. The corrected image can then be used to further finetune (retrain) the model.
You can correct errors in one of two ways:
Delete a mask by holding down the ‘control’ key and clicking on it.
Draw a new mask by right-clicking anywhere in the image and tracing an outline, ending where you began to draw.
Try correcting some of the segmentations. It might be easier if you switch between masks and outlines (use ‘z’ and ‘x’ as explained before).
Once you are happy with your corrected masks, take a look in the folder containing all of the training images. You will notice there is a new file there, called ‘hard\train\104_img_seg.npy’. This contains your corrected segmentation and will become a new bit of ground truth used during finetuning the model. But… how do you start this finetuning step?
In Cellpose, start: `models →
train new model with images and masks in folder’.
You should see a window like this one:
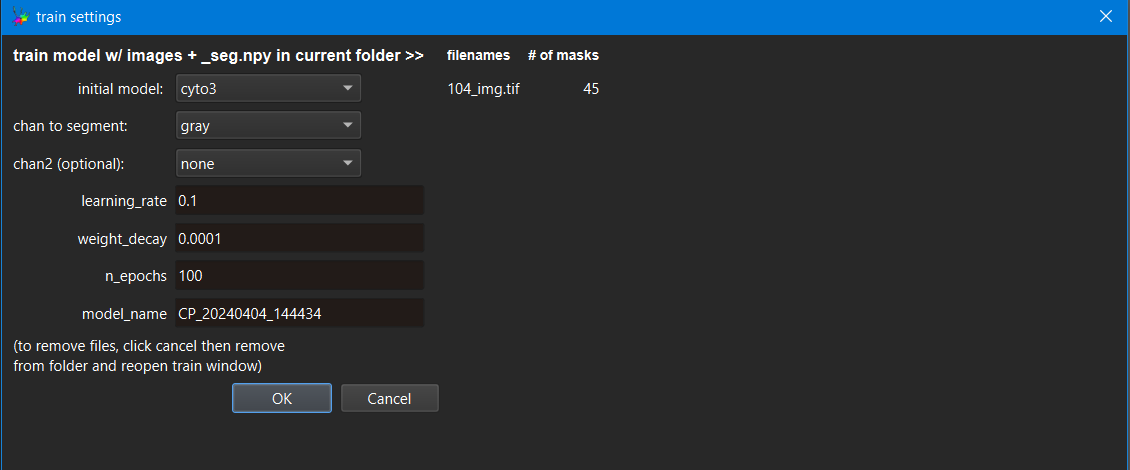
First, we need to select which initial model to use (in the screenshot
above, we are retraining the cyto model (but of course you may choose
to retrain any available model). You can, and should, give your new
model a name. You can also see which (corrected) images you are going to
retrain the model on under ‘filenames’, and the number of masks that
will be used for retraining in that image. Click OK whenever you are
ready to retrain and finetune the selected model!
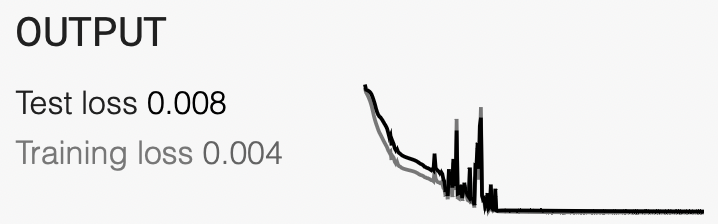
During training you should see something like the following if you check the
console (where you started cellpose from). What is going on here? Remember
back the lecture when we discussed training steps and epochs.

Once done, Cellpose will open the next image in the folder and automatically use the freshly finetuned model to segment it (NOTE: in cellpose 3 there seems to be a bug where the new model is not being used to segment the newly loaded image. If you notice the segmentation isn’t very good, manually select your newly trained model under the “other models” dropdown and run it). You can now repeat this process as often as needed. Cellpose will in each iteration finetune the same original model, but will do so with an ever increasing number of user labeled masks (the ones you have created). Eventually you will either loose hope or find that Cellpose’s prediction become good enough for you to be 😻!
Once you are happy with the results you are getting, apply your final model to the test data we have segmented at the start of the exercise (importantly: your model has not previously seen this image during finetuning! Why is this important again?). Is the result better than with the initial model you started with?
Using the image restoration functions#
Cellpose also has some ability to restore images by denoising and deblurring. This is used to aid the segmentation of noisy data. Let’s test it!
From the folder you downloaded earlier, open the “noisy” folder and open “convollaria.tif” in cellpose.
Try using the Cyto3 model to segment this image (you can leave the diameter at 30 pixels).
Not a very satisfying result is it?
This is partially because the data is very noisy. Let’s try to add some denoising befre we segment.
Under “Image restoration, press the “denoise” button. What do you notice about the image?
Try using the same parameters and mode to segment this image. Did it improve?
Try some other restoration modes. Try using some custom filters and see if you can improve the segmentation. What might be useful for denoising this image?
Tip
Want to use Cellpose when you get home, but having trouble with the conda installation? You have (at least) a couple of potential options!
Note
As of April of 2024, both of these are still using Cellpose 2, which does have human-in-the-loop retraining but not denoising or image restoration
EMBL has the Bioimage ANalysis Desktop (BAND) program, which allows you to check out virtual machines in the cloud. You simply visit a website, tell them the resources you need, and get a machine with >20 helpful image analysis tools pre-installed.
Upsides: No installation, everything is correctly configured and ready to go, simulataneous access to lots of tools at once, you can ask for machines with GPUs
Downsides: They have limited capacity and sometimes machines aren’t available due to EMBL courses. You have to upload your data to their servers, and download your results from them.
Cellpose can be used with CellProfiler, both in Python if you have both programs
piporcondainstalled, but CellProfiler also offers a way to use a pre-built version using Docker 11Upsides: You can use Cellpose models without ever touching your terminal, and while keeping your data local - you only need to install CellProfiler and Docker
.appor.exefiles from their respective websites, download the plugin, and then point CellProfiler at the location of the downloaded file.Downsides: this only lets you run pre-trained Cellpose models, not train your own. Running Cellpose in CellProfiler via Docker is also MUCH slower than running it when installed via Python (though it will mostly be compute time, rather than human time, once you’re in analysis mode and running all your images unsupervised). You’re limited to the hardware you have locally.
Exercise 3: First steps with Google Colab (don’t waste too much time here…)#
The following steps should get you started in no time:
Please go to https://drive.google.com and log in with your google account. If you do not have (and do not want one), please team up with somebody else who has one(or is willing to create one).
If you never used Google Colab:
click on
 >
More > Connect more apps
>
More > Connect more appssearch for “Colaboratory” and connect it to your google account
You should now see a new folder in your google drive:
Now let’s look at some existing tutorial notebook and play a bit with it:
Open https://colab.research.google.com/notebooks/intro.ipynb and try to execute the few code cells you’ll find there.
Now open a linear regression example available online and follow it until (and including) the section “Simple Linear Regression”.
Don’t waste your time today to go any further… it is of course super interesting, but totally out of scope… ;)
https://colab.research.google.com/github/jakevdp/PythonDataScienceHandbook/blob/master/notebooks/05.06-Linear-Regression.ipynb
Credit:
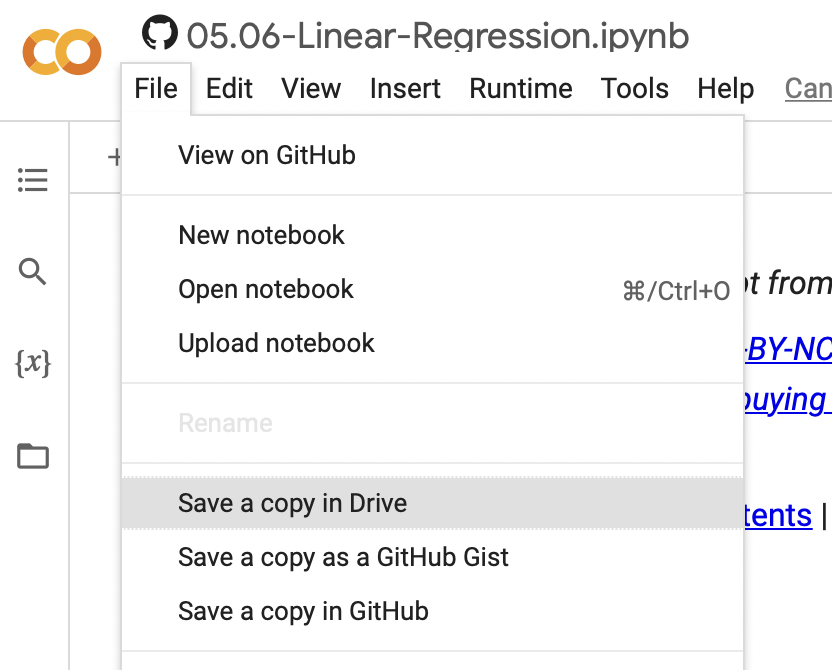
Note: you can save your own copy of this notebook on your own Google Drive via…
Exercise 4: First steps with ZeroCostDL4Mic#
ZeroCostDL4Mic12 is a collection of (hopefully) self-explanatory Jupyter Notebooks for Google Colab. They are meant to quickly get you started on learning how to use deep-learning methods specifically created for microscopy image analysis.
Google Colab itself provides the computations resources needed and does so at zero cost. ZeroCostDL4Mic is designed for researchers that have little or no coding expertise to quickly test, train and use popular neural networks approaches.
Scroll down and be amazed by the amount of available methods… ;)
You could pick any method now and start playing with… but… why not start with “Noise2Void (2D)”?
Find it, download any example data, then click on “Open in Colab”. (Hint: the sample data can also be found behind the “Lab Data” link above…)You will find yourself at a page looking roughly like this:
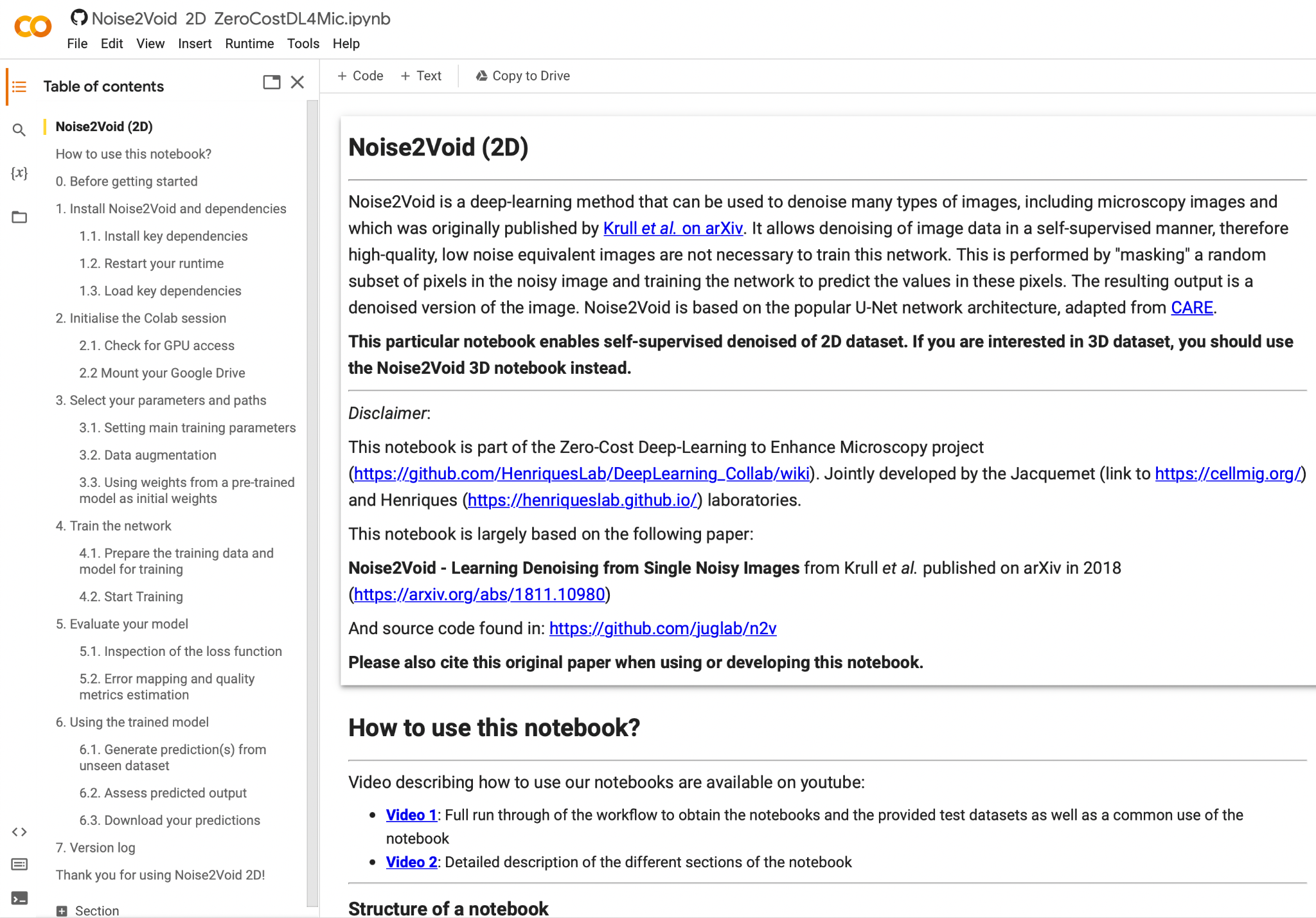
In order to work on your own data (or also on the test data we downloaded just before), please upload it on your Google Drive (in any folder you’d like).
While uploading, you can start going through the Noise2Void notebook we opened before.
a. At some point (at Step 2.2) you will be asked to connect to your Google Drive. Please do so! 🙂
b. Note that you will now see the content of your google drive. Click first on the “Files” icon, then go one folder up, and you should see something like this:
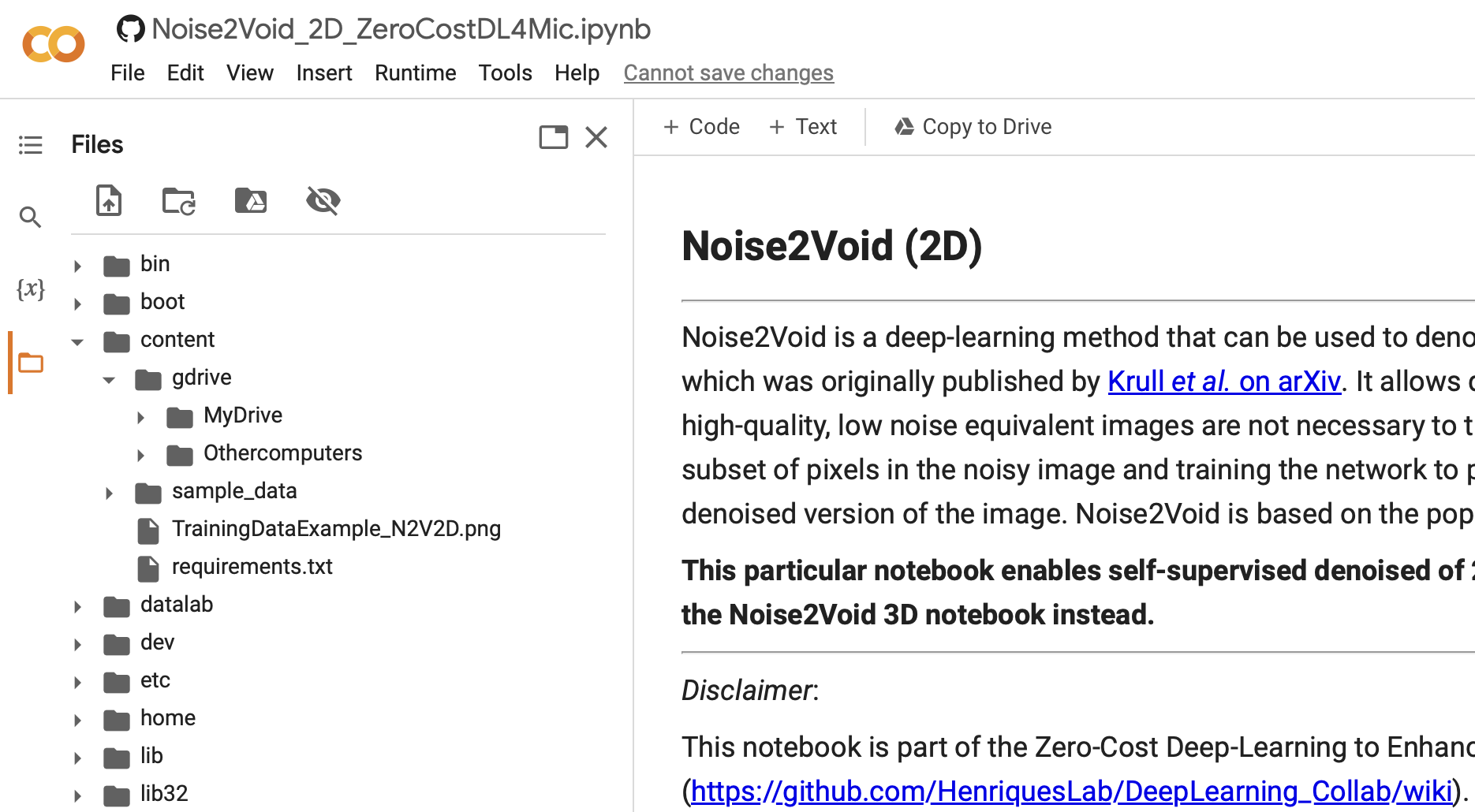
c. DONE! Let’s continue in the next exercise…
Exercise 5: Image Denoising with Noise2Void (in “Zero”)#
In this exercise we will denoise some data with Noise2Void. Please remember, Noise2Void is removing pixel-noises only (Poisson noise, readout noise, etc.).
If you do not have really noisy data at hand, feel free to use the example data offered on the ZeroCostDL4Mic wiki (or from within the Lab Data link from above, or just use https://tinyurl.com/DLLab-droso2d). Ideally, as a group we use a bunch of different datasets, so we have more results to look at…
Decide what data to use (see remarks above).
Copy the data to any sensible place on your Google Drive.
(Why? Data on Google Drive can be read easily and FAST by Collab…)Unfortunately, there is a bug in the published version of N2V in ZeroCost. Here you will find a copy of the notebook where we have fixed the bug. Be sure to make your own copy of this notebook in your drive (“File” -> “Save a copy in Drive”) before you start
Go through the notebook. The first time around this will take a while and be confusing at times. Plow through it, you will soon be happy!
Ask questions, help each other!a. Pro tip: if you are in a hurry, reduce the number of epochs to some small number, e.g. 10 or 20. Results will be much worse, but you can always crank that number up later…
b. Later today, when you are done with the exercises, you might want to re-run your favorite notebook with the suggested number of epochs. Collab will work while you have fun on your free evening… 🙂
Bonus Exercise: Classifying images in the browser in Piximi#
Piximi 13 is a web app currently in development for training and running deep learning models in your web browser. Under most circumstances (with Cellpose as the major exception), all compute happens locally - you load your images into your web browser, but they are NOT sent to the internet, they stay locally on your machine. While this has some disadvantages (namely, that you’re limited to the resources on your own machine), this means you get the benefit of web applications (namely, no need to install anything) but don’t have to worry about upload times or where in the cloud your data is stored.
Piximi will eventually include 3 major functionalities - Segmentation and Object Detection, Classification, and Measurement. In order to train deep learning models for object detection and segmentation, it also includes an annotation tool. As of April 2024, you can train your own classification models, annotate images, and run pre-trained object detection and/or segmentation models that we provide; we expect measurements to come out by end of Q2 2024 and hope to provide trainable segmentation by the end of 2024. You can keep visiting piximi.app to see what’s available!
Train a 10-class classification model using MNIST#
Piximi is designed for biologists but can be used on non-biological images as well. Here, we’ll use 1,000 handwritten digits from the classic MNIST dataset 14 , which consists of cropped images of digits from old census data and high school students.
Train a classifier#
Tell Piximi you want to open an example project

Scroll through the images - you’ll see that most are categorized as a particular digit, but about 60 have been intentionally left un-categorized for testing purposes.
Are there any categorizations you aren’t sure about or disagree with?
Tell Piximi you want to fit a classifier for these images
You will now see Piximi’s training dialog; you can choose to tune some of the hyperparameters before training (though we’ve chosen here reasonable defaults that should work well). Otherwise, hit
 to train.
to train.After an initialization step, you will see a performance chart that looks like the below, as well as a loss graph. You can keep hitting
Fit Classifierto keep adding more epochs of training.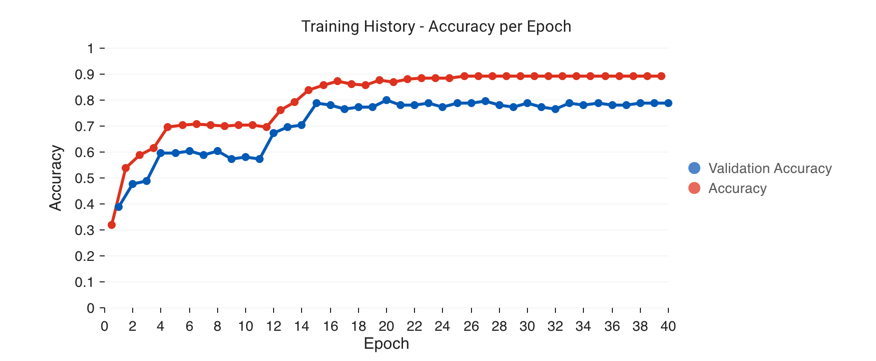
Are you overfitting? How can you tell?
Evaluate your classifier#
Once you’re satisfied with your training (either because it’s great or because you’re satisfied that it has plateaued), close the training dialog. Hit the Evaluate Model button to check your confusion matrix.

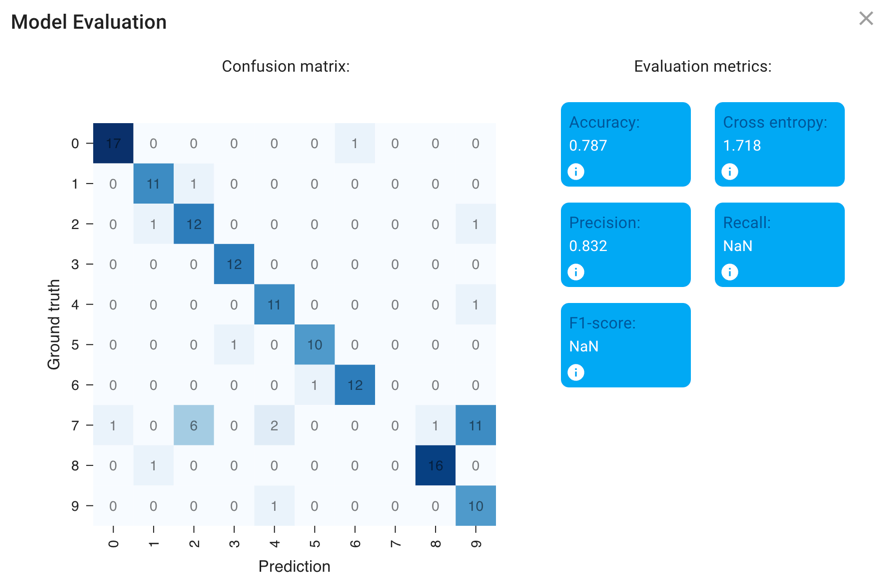
Questions for you
What patterns of mistakes do you notice? Are they the kinds of mistakes you would expect?
A confusion matrix helps you figure out patterns of mistakes, but it can only tell you about the performance of your model on data for which you’ve already provided the answer - it can’t tell you about performance in your unlabeled data. It is critical then to always apply your classifier to new, unseen data to see how it performs.
Important
It is quadruple-extra critical when only a small fraction of your data is labeled, which is NOT true here but is often true in biological situations and you may hope will be true in your future work (after all, if you have to hand label almost all of your data, then what’s the point of training a model?)
Hit the
Predict Modelbutton to apply the model to the unlabeled data
Evaluate the performance of the predictions - you may find that hitting one or both of these buttons helps you do that


Fix (some?) mistakes#
If and when (when), you find some errors in the predictions, you can fix them by assigning them a new category.
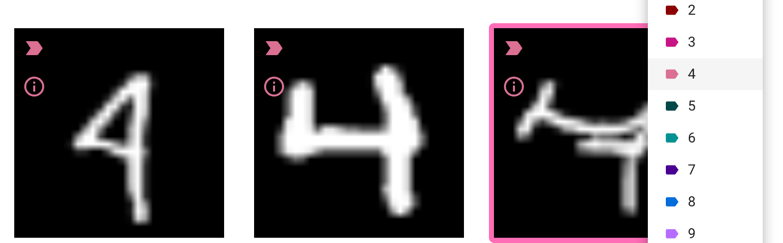
Depending on why you’re using machine learning, you might choose to fix all the wrong images at this stage, or only some
Is your goal to just get the classifications right and then use them for something, and most of them are?
In that case, there’s no harm in just fixing the few mistakes and then moving on to other downstream quantification steps (coming soon!).
If this is your goal but there are a lot of mistakes, you might not choose to fix all of them at this stage, but just fix a subset and then try to train again so you can get to a point where the errors are at a small enough level that you CAN do final data cleaning by hand
Is your goal to create a robust, reusable classifier to use on other sets or in other contexts?
In that case, you might want to fix only a subset of the mistakes before retraining, so you can get a sense of if your model performance is improving.
If retraining, once you’ve done your chosen recategorizations, clear predictions (
 ) and then hit fit again.
) and then hit fit again.
Important
If this is indeed your goal, you need to have some test unseen data somewhere else that you are not tuning on here! Once you’ve run any version of your model, at any stage, on unseen data, that data is now “seen data”, and can’t be used as a test set anymore. How you plan your data splits (and how much, and which, data you keep locked away as test set(s)) is critical to any kind of machine learning research
Save things for later#
Reproducible science matters! You can therefore save your Piximi project file for later, as well as save your model for later use. You might find the former handy if you want to add more data later, and/or you just want to confer with someone else (including a paper reviewer, or future you) about how difficult data points were handled.

Train a 3-class classification model on U2OS cells#
Note
Sometimes this data set takes a long time to load, sometimes it doesn’t! We’re not sure why. Feel free to skip it if it’s taking a long time and being annoying.
This data set is in some ways more challenging, but also shows a more biologically relevant classification scenario, alongside the ability to do more human-in-the-loop retraining since, unlike MNIST, the majority of the data is NOT already categorized for you.
Refresh Piximi, and then load the U2OS-cells cytoplasm crops dataset

Optional: fix how the images look
You need not do this, since it can be a bit slow, but it is necessary if you want to assess the performance of the no-GFP class (and will make things much easier if you are red-green colorblind).
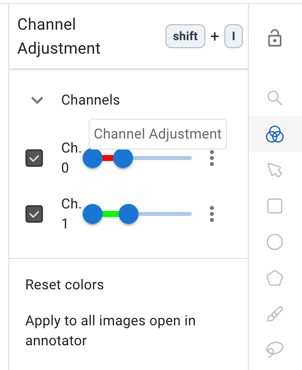
Piximi’s current defaults are to load two-channel images as red and green, and to rescale each image min-max individually. While we work to fix those bugs, here’s how you can manually set the colors to something better (and more uniform)
Use human-in-the-loop classification to train a high-performing 3 class classifier. How high can you get the evaluation metrics? How many rounds and how many corrected classifications does it take you to get there?
Bonus Exercise: Use Fiji’s Noise2Void Plugin#
In this exercise you will use a Noise2Void plugin in Fiji. You will have to install it first.
Open Fiji.
Go to Help - Update… - Manage update sites, then check the CSBDeep update site and say Close - Apply changes.
Restart Fiji.
Download https://tinyurl.com/DLLab-droso2d and open this 2D+t tiff in Fiji.
Start the “N2V train + predict” plugin.
Figure it out… 😉
a. Please ask question at ANY time!
If all works out ok, you will see something like…
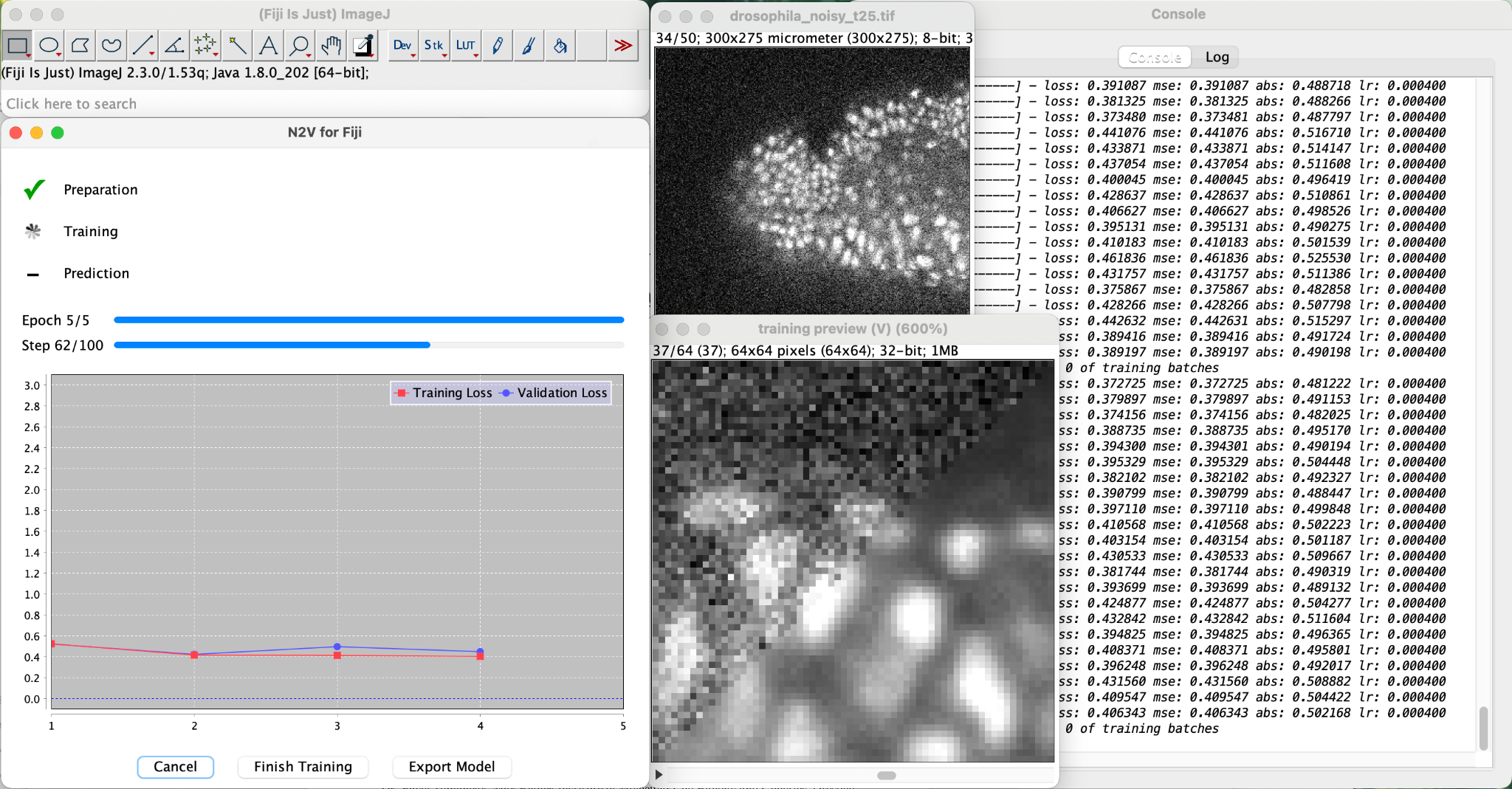
The training will likely be VERY slow, but to sweeten up the wait, we show you a nice preview.
Together with the result you also get the trained model for later reuse.
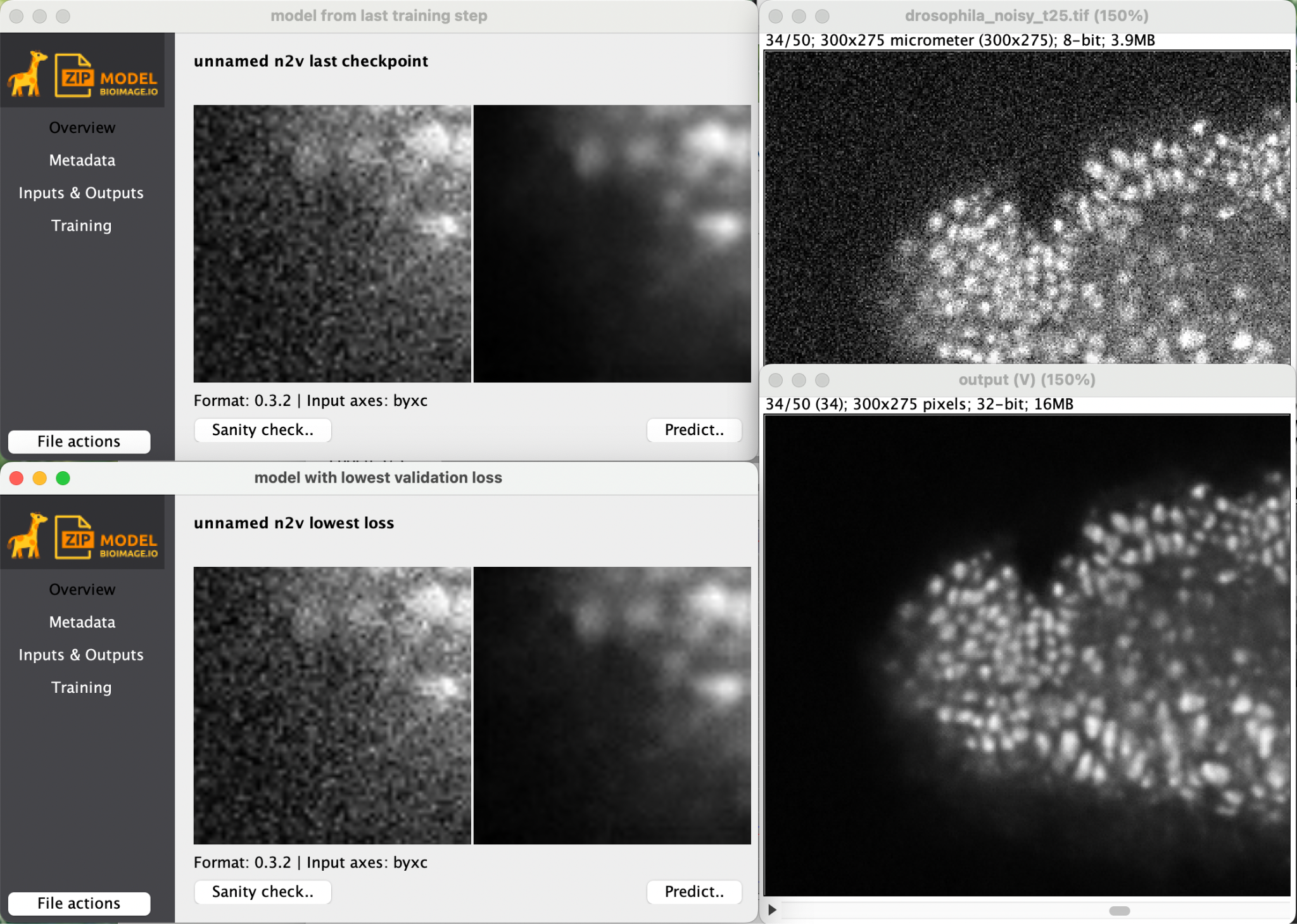
Try to use the trained model to denoise the same stack or any other image of your choosing.
Tip
Be sure to set the axes correctly. On the same stack, this will require you to add a third dimension which contains multiple time points. What should we use as the third axis and why is it ‘B’ when you use the same stack you used for training?
Bonus Exercise: Image Segmentation with StarDist (in “Zero”)#
Now that you have experienced how to use ZeroCostDL4Mic Collab notebooks, switch it up, do some instance segmentation! We suggest the StarDist notebook, but if you feel adventurous, choose something else you find most interesting in the context of your own research.
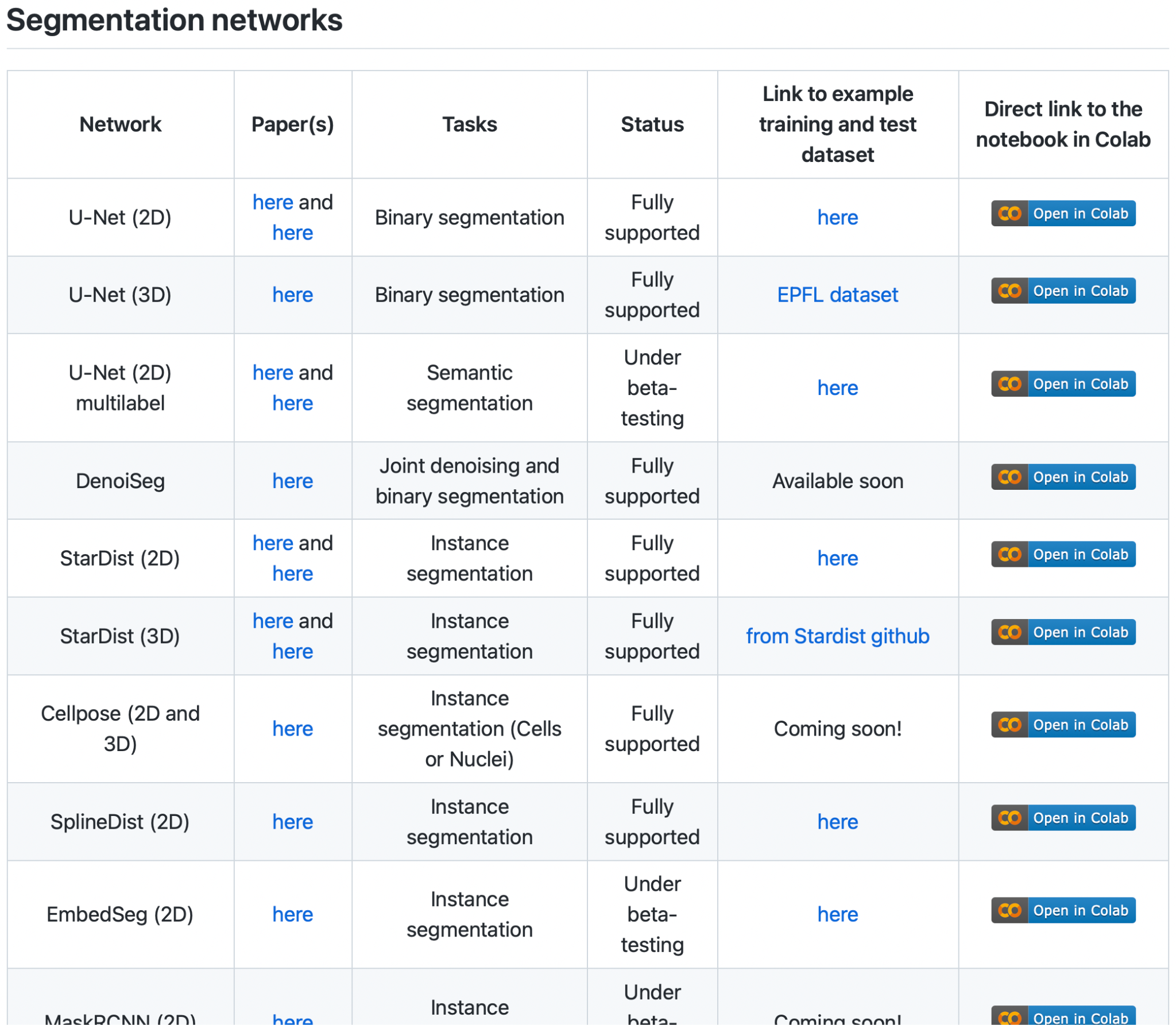
Go through the notebook you chose. And again:
Ask questions, help each other!
Tip
Reduce the number of epochs to some small number to save yourself long waiting times!
Later today, when you are done with the exercises, you might want to re-run your favorite notebook with the suggested number of epochs. Collab will work while you have fun on your free evening… 🙂
Super excited about deep learning now and want to know where to find the latest models?
The Bioimage Model Zoo 15 contains a number of deep learning models, applications, and example data sets you can use on your own data or to train your own network.